In 2015, approximately 438,000 deaths were due to malaria, with 90% of those deaths occurring in Africa and 80% occurring in under fives. Whilst these rates have been steadily decreasing year by year, the emergence of resistance to current drugs threatens to prevent control and eradication efforts. It is therefore crucial that we monitor and understand how resistance emerges within parasite populations to ensure that medications remain functional.
Emerging resistance has already removed drugs from our arsenal. Take, for example, chloroquine and similar quinine-derived therapies. Before the turn of the millennium these were the standard front-line drugs used to kill malaria parasites, but over-use led to resistance, today you’re more likely to be treated with an artemisinin-based combination therapies (ACTs) instead.
Broadly this chloroquine to ACTs trend holds true across the globe but there are exceptions. Malawi, for example, was an early adopter of a combination therapy consisting of sulfadoxine and pyrimethamine (SP). This remained the Malawian drug of choice for fourteen year until 2007 when Malawi became one of the last countries in Africa to adopt ACTs. This period of sustained SP use have left its mark on the local parasite population with resistance to SP being near omnipresent whilst resistance to chloroquine is effectively absent.
SP resistance stems from variants in two parasite genes (dhps and dhfr), the proteins which SP targets. Sulfadoxine (the ‘S’ of ‘SP’) targets DHPS, whilst pyrimethamine (the ‘P’) targets DHFR, both proteins are members of the parasite folate pathway. In essence, if the parasite were a factory in which one assembly line produces folate, S and P would be two saboteurs who each know how to break two specific machines (DHPS and DHFR). Slight changes to either machine will prevent S and P from dismantling them, thus allowing the assembly line (and by extension the parasite) to continue unharmed. This relatively complex resistance contrasts with that of chloroquine, whereby only a single mutation in the crt gene, known as K76T, conveys resistance.
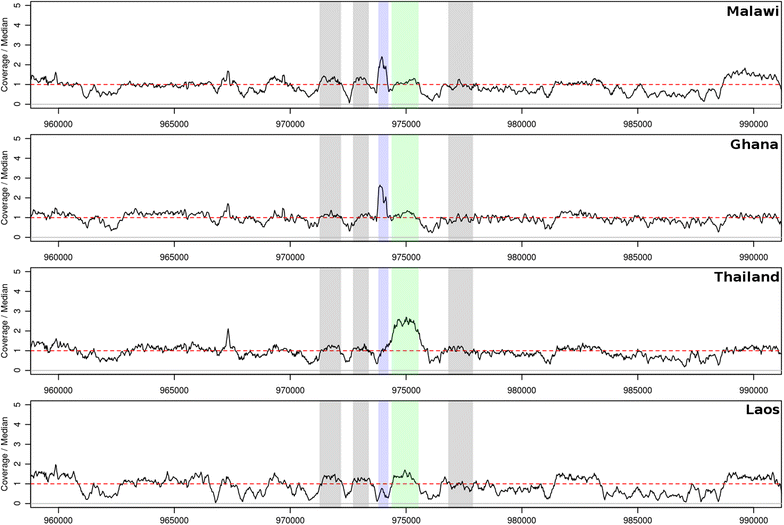
So why not just install saboteur-proof versions of DHFR and DHPS? Efficiency. Whilst these alternative machines may be the best option when S and P are present, they might produce less product than the original versions. This is where a third machine/protein, known as GCH1, comes in. GCH1 lies ahead of DHFR and DHPS in the folate assembly line, and some factories have benefited from multiple copies of the machine. For the parasites, we have examples of this happening in the wild. Specifically duplications of gch1 have been associated with resistance in Southeast Asia, particularly Thailand, and could increase the levels of GCH1 to compensate for resistance-associated forms of DHFR and DHPS.
So the solution seems simple, if the parasite is in a region of high SP use it should benefit from not just variants of DHFR and DHPS but also duplications of GCH1. But whole gene duplication is not necessarily the only way to increase the amount protein from a gene. Our recent research in Malawi has identified a 436bp duplication of the gch1 promoter, a gene’s on/off switch, rather than the whole gene. Whilst the full functionality of this variant remain to be explored in the lab, the highly specific signals are extremely note-worthy. If functional in a similar fashion to the gch1 duplication hypothesis above, these variants may act to ‘lock in’ SP resistance, reducing the reemergence of SP susceptibility in contrast to the relatively speedy removal of chloroquine resistance. Such variants may therefore undermine newer forms of malarial treatment which utilise SP, such as intermittent treatment during pregnancy (ITPp) and seasonal malaria chemoprevention (SMC).
This blog was inspired by research published in Malaria Journal: http://dx.doi.org/10.1186/s12936-016-1634-6